Resources
Aug 03,2021
Newsletter n° 16 WHD Case: PT ∣ Assessment of Novelty and Inventiveness for Pharmaceutical Preparation Invention
Tianjin Xinchen Techfields Pharmaceutical Technology Co., Ltd. owns the patent for invention ZL200680055379.X, entitled “Positively Charged Water-Soluble Prodrugs of Ibuprofen with Very Fast Skin Penetration Rate”. Clinically, Ibuprofen is known to be widely used as a non-steroidal drug for analgesia, antipyretic and anti-inflammation. Ibuprofen is usually administered orally, but it may cause a number of side effects, such as indigestion, stomach and duodenal bleeding, gastric ulcers or gastritis. The patent at issue, which administers an ibuprofen prodrug transdermally, effectively avoids the side effects caused by oral administration of ibuprofen and is superior to oral administration of ibuprofen in terms of efficacy. The patented preparation is in the clinical research stage in China.
In practice, an invention of pharmaceutical preparations is generally considered to be less creative than that of an invention creation of compound. It usually puts the patentee of a pharmaceutical preparation invention in a disadvantageous position in defending the validity of its invention.
Case Brief
Claim 1 of the patent at issue seeks protection for a transdermal therapeutic application system containing ibuprofen prodrugs of Structure 1 administered in the form of solution or spray. Below is the structural formula of the ibuprofen prodrugs, consisting of Ibuprofen residue (portion in red box) and a modifying group (remainder of the formula).
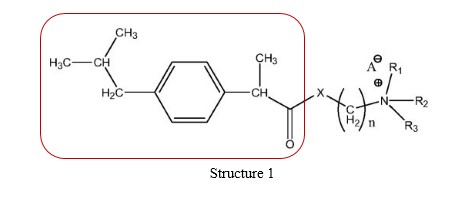
The patent description explicitly records the advantages of administering the prodrug transdermally over administering the parent drug ibuprofen orally. Compared with ibuprofen, the prodrug has considerably improved water solubility and skin diffusion rate, which enables it to penetrate skin barriers fast and effectively and avoids side effects attributed to oral administration of ibuprofen. In the meantime, the transdermally administered prodrug is remarkably superior to the orally administered ibuprofen in terms of therapeutic effects.
On February 13, 2018, Mr. Qi filed a request for invalidation of the patent by challenging the clarity, support, novelty and inventiveness of the claims. The China National Intellectual Property Administration (CNIPA) maintained in its invalidity decision No. 38911 on January 30, 2019, the validity of the patent on the basis of the revised claims. Mr. Qi appealed the invalidity decision to the Beijing IP Court, narrowing down his invalidation grounds to novelty and inventiveness of the claims. On July 20, 2020, the Beijing IP Court rendered a decision to uphold the invalidity decision. The judgment has become effective.
Court decision
1. Regarding novelty
Evidence 2 discloses an isotonic drug solution containing the compound BF-DEAE (a compound falling within the structural formula 1 as defined in Claim 1), whose anesthetic effect has been tested by dripping it onto the corneal surface of guinea pigs. As to the novelty of Claim 1 relative to Evidence 2, the court affirms:
(1) As far as the classification of pharmaceutics is concerned, transdermal preparation and eye drops are different dosage forms;
(2) The purpose of the invention is to improve the penetration rate of Ibuprofen to biomembrane and skin barrier so that it can be delivered transdermally, thereby avoiding the side effects of Ibuprofen. Nevertheless, the eye drops in Evidence 2 is applied to cornea, which consists of connective tissue rather than skin and is devoid of cuticle barrier. Therefore, the "transdermal therapeutic application system" of Claim 1 does not include eye drops.
2. Regarding inventiveness
Evidence 7 discloses Ibuprofen prodrugs in ester form, e.g. Compounds 36 and 37, which demonstrates superior topical anti-inflammatory activity to Ibuprofen in the topical use pathway. Thus, the difference between Claim 1 and Evidence 7 is that the compound in Evidence 7 fails to form salt, whereas the compound in Claim 1 is in the form of salt. Evidence 3 discloses a testosterone prodrug with the same modifying structure as in Claim 1, with a transdermal rate 60 times faster than testosterone per se.
As for the technical problem actually solved by Claim 1, the court analyzes from the following aspects:
(1) Evidence 7 tests acute toxicity and pharmacological activity (including anti-inflammatory and analgesic activity) of compounds. The results indicates that Compounds 36 and 37 show similar anti-inflammatory effects to Ibuprofen when administered orally, and slightly superior anti-inflammatory effects (1-2 times higher) to Ibuprofen when administered topically. However, Compound 36 is inferior to Ibuprofen in analgesic efficacy and acute toxicity, and Compound 37 is slightly superior to Ibuprofen in analgesic efficacy, but inferior to Ibuprofen in acute toxicity in oral administration.
(2) Evidence 7 records that "some of the compounds of the present invention have high degree of pharmacological activity and low toxicity and, in particular, the topical application of the compounds produces stronger anti-inflammatory effect than Ibuprofen".
(3) In fact, Table I of Evidence 7 discloses the structures of 66 compounds, and Table II of Evidence 7 discloses the pharmacological effect and acute toxicity of 30 compounds. In Examples, the preparation methods of some compounds are disclosed, but all the compounds disclosed are esterified derivatives of carboxyl groups in Ibuprofen structure, including alkyl esters, aryl esters and amino esters, among others. Based on the pharmacological activity and acute toxicity data exhibited in table I, the amino ester derivatives 36 and 37 are not superior to the alkyl ester and aryl ester derivatives, and compounds 36 and 37 are not even mentioned in the preferred examples for the preparation method.
(4) In a nutshell, Evidence 7 provides the esterified derivatives of Ibuprofen to achieve the technical effect of reducing toxicity and improving anti-inflammatory potency in topical application, rather than providing prodrug to improve solubility, safety, and skin penetration. Therefore, the technical problem actually solved by Claim 1 is to provide a safe and high skin penetration Ibuprofen prodrug preparation.
As for the combination of Evidence 7 and Evidence 3, the court affirms:
(1) the prodrug of testosterone disclosed in Evidence 3 is substantially different from that of Ibuprofen in this patent.
(2) Propranolol, scopolamine, benzocaine and lidocaine mentioned in Evidence 3 all contain lipopilic parts and amino group in their own structures and do not need prodrug modification. Moreover, Evidence 3 simply mentions that "using similar tert-amino group" to modify indomethacin and deoxycorticosterone results in a 6-20 times increase in in vitro transdermal flux, but fails to specify how to modify them.
(3) Evidence 3 discloses that in vitro human skin penetration rate of testosterone prodrug is about 60 times faster than that of testosterone, its in vivo penetration rate is 7 times of that of testosterone, and in vitro transdermal flux of derivatives derived from modified indomethacin and deoxycorticosterone increases by 6-20 times. The charged prodrug Ibuprofen in this patent, however, has a 250-fold difference in skin penetration rates in vitro and a 60-fold difference in vivo when compared with Ibuprofen, significantly exceeding the scope that could be reasonably expected by a person skilled in the art based on the content of Evidence 3.
(4) To sum up, Claim 1 is non-obvious relative to the combination of Evidence 7 and 3 and has achieved unexpected technical effect, and thus involves an inventive step under Article 22.3 of the Patent Law.
Wanhuida Intellectual Property represents the patentee in both proceedings.
Comments
The decision of the Beijing IP Court has referential significance for assessing novelty and inventiveness of pharmaceutical preparation invention. As regards the assessment of novelty, the court takes a holistic approach in considering the definition effect of all technical features in the claim, including that of the subject matter title per se on the product type. As regards the assessment of inventiveness, it analyzes in detail whether there is technical teaching in the prior art as a whole, which aligns with the practice in the examination of inventiveness that the obviousness of claim should be assessed by taking the prior art as a whole.
(1) The Patent Examination Guideline provides that in the assessment of novelty, whether the invention is found to be identical with the prior art hinges on whether the said invention is substantially the same with the prior art in terms of technical field, the technical problem solved, the technical solution and the desired effect. The court decision follows the aforesaid examination principles, which not only focuses on the differences between the technical solutions per se, but also clarifies the substantial differences between the invention and the prior art from the technical field, the technical problem solved, and the technical effect, which is an effective rebuttal to the plaintiff’s biased approach, which merely dwells on the structure and composition of the product.
(2) The assessment of obviousness hinges on the determination of the technical problem actually solved by the invention. To all appearances, pharmaceutical preparation invention is slightly different from the prior art, and the distinguishing feature is likely to be misconstrued as common knowledge or conventional technical means in the field. In this case, the only difference between Claim 1 and Evidence 7 is that Claim 1 uses the compound in the form of salt instead of ester, however, the invalidity decision and the court decision unanimously elaborate on the technical effect that the distinguishing feature could achieve in the technical solution of the invention based on the disclosure of Evidence 7 and the effect recorded in the patent, so as to identify the technical problem actually solved by the invention.
(3) In terms of the assessment of obviousness, it has become a trending practice to take the prior art as a whole in identifying technical enlightenment. In respect of the disclosed contents of Evidence 3, the plaintiff points out mere fragments of the evidence, thus it is unconvincing to determine whether the technical solution of the patent at issue is obvious relative to these contents alone. The Beijing IP Court, based on the evidence adduced by the patentee, not only takes into account the difference between the testosterone prodrug disclosed in Evidence 3 and the technical solution of the invention, but also demonstrates the teachings given in Evidence 3 in combination with the structures of other compounds disclosed in the evidence, which leads to the finding that the patented technical solution is not obvious.
(4) The actual technical effect of the invention shall also be factored in the determination of non-obviousness. This is also consistent with the interpretation on the relationship between unexpected technical effect and non-obviousness in the Patent Examination Guideline: "When an invention produces unexpected technical effect, on the one hand, it indicates that the invention has significant progress, and on the other hand, it also reflects that the technical solution of the invention is non-obvious". The court, in assessing non-obviousness, takes into account the actual technical effect of the invention. The practice could serve as a point of reference in similar cases.
Authored by Dr. Wu Xiaoping
